About
Ovarian Imaging in Animal Models
|
The overall goal of our project is to develop two capabilities critically
needed in the fight against ovarian cancer: 1) a rodent model of ovarian cancer
that closely resembles the human disease, and 2) minimally-invasive imaging
modalities sensitive to early neoplastic changes. The long-term outcome of
our research is expected to be the identification of early neoplastic changes
that predict ovarian cancer. In a study of 83 rats we found that the application of the industrial
chemical VCD was effective in eliminating all primary and primordial
follicles and thus inducing menopause in a mechanism similar to what occurs
in women. Most ovarian cancer occurs in post-menopausal women.
The combination of VCD with the carcinogen DMBA caused tumors in 57%
of the rats at 5 months after dosing with DMBA [Hoyer et al., 2009]. Analysis
of another 48-rat study is ongoing. In this study, two additional categories
(control/DMBA/estrogen and VCD/DMBA/estrogen) were added. If histopathological
analysis on the last ovaries follows early trends, estrogen appears to be
protective against development of Setoli-Leydig ovarian tumors. In technology development, we developed a focused OCT/LIF probe which will allow higher resolution imaging in both the OCT and fluorescence emission channels. We have also developed the first-ever OCM handheld probe. This probe has a 6 mm diameter tip, so that surgical imaging of the ovary can be performed with only a small incision necessary. The OCM probe has excellent characteristics, providing 3 µm later resolution, a 1 mm en face field of view, and 600 µm imaging depth adjustment [Korde et al., 2009]. A photograph of the probe is shown in figure 1, and it is ready to be used in the next imaging study.
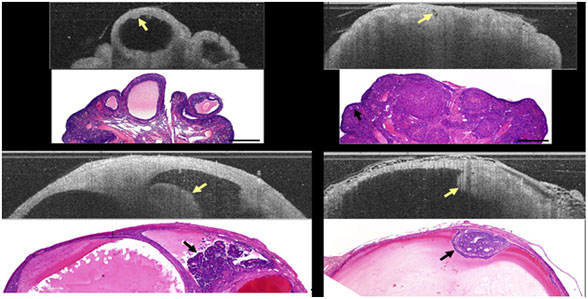
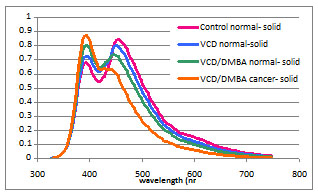
Figure 1. OCM endoscope. The distal portion is 6 mm diameter. Figure 2 presents a compilation of normal cycling, VCD-treated post-menopausal, and neoplastic ovaries. Image characteristics for follicles, corpora lutea, follicular remnant degeneration, cysts, and neoplastic regions have been defined. LIF emission spectra show a distinct trend towards higher 390 nm emission and lower 450 nm emission with progression from normal cycling, VCD treated, VCD/DMBA treated non-neoplastic, to VCD/DMBA treated neoplastic (figure 3). The use of the mouse ovary introduces challenges and opportunities due to its small size. Optical coherence imaging is more challenging, but recent advancements in OCT/OCM resolution mean that we are able to obtain images of high quality. We have been intrigued by changes in the stroma texture and grayscale attenuation with disease that are seen in the optical coherence images. However, even OCM does not have the sub-micron resolution necessary to visualize collagen bundles and subcellular structures.
Figure 2. Example images from rat ovary study. Top left: normal cycling ovary OCT and histology image. Arrow points to an egg inside an antral follicle. Top right: VCD treated ovary OCT and histology image. No follicles are seen but an abundance of corpora lutea. Follicular remnant degeneration is seen a small hypointense regions (arrows). Bottom left and r ight: neoplastic ovaries OCT and histology images. In both, tumor is seen as an irregular protrusion into a cyst. Lower images are 5 mm in length by 1.5 mm depth, others to scale. Among the opportunities presented with the mouse ovary is the possibility of using microscopic imaging. We have recently completed development of a custom and modular advanced intravital microscope, sponsored under a shared instrument grant (S10RR023737) and ideally suited for ex vivo and in vivo imaging of mouse ovary. The microscope is capable of simultaneous two‐photon excitation, second harmonic, and confocal imaging. A single‐channel time‐correlated single photon counter is used to collect fluorescence lifetime data. A large motorized stage provides support for intravital small animal imaging or on‐stage incubation of engineered tissue constructs. The x‐y movement of the stage is motorized and the course z‐position is adjusted hydraulically over 5cm to match the sample thickness. In one configuration, the system is optimized for imaging of endogenous cellular fluorescence and SHG at 760nm excitation, with SHG occurring below 400nm, NADH auto fluorescence between 400 and 500nm, and emission of flavoproteins between 500 and 600nm.
Figure 3. LIF spectra of normal cycling, VCD treated, VCD/DMBA treated normal, and VCD/DMBA treated neoplastic ovary showing a continuous progression towards lower 450 nm emission intensity. References Brewer MA, Utzinger U, Barton JK, Hoying JB, Kirkpatrick ND, Brands WR, Davis JR, Hunt K, Stevens SJ, Gmitro AF, “Imaging of the ovary,” Technol Cancer Res Treat. 3(6):617-27, 2004. Hariri LP, Bonnema GT, Schmidt K, Winkler AM, Korde V, Hatch K, Brewer M, Barton JK “Laparoscopic optical coherence tomography imaging of human ovarian cancer,” accepted for publication in Gynecologic Oncology, 2009. Hoyer PB, Davis JR, Bedrnicek JB, Marion SL, Christian PJ, Barton JK, Brewer MA, "Ovarian neoplasm development by 7,12-dimethylbenz[a]anthracene (DMBA) in a chemically-induced rat model of ovarian failure,” Gynecological Oncology, 112:610615, 2009. Kirkpatrick ND, Brewer MA, Utzinger U, “Endogenous optical biomarkers of ovarian cancer evaluated with multiphoton microscopy,” Cancer Epidemiol Biomarkers Prev. 16(10):2048-57, 2007. Korde VR, Liebmann E, Barton JK, “Design of a handheld optical coherence microscopy endoscope,” Proceedings of the SPIE 7172:71720D, 2009. Skala, M., et al., In vivo Multiphoton Fluorescence Lifetime Imaging of Free and Protein-bound NADH in Normal and Pre-cancerous Epithelia. Optical Society of America: Biomedical Topical Meeting, 2006. |